How Many Valence Electrons Does a Neutral Magnesium Atom Have
There will be two electrons in the first energy level eight in the second level eight in the third level and one in the final energy level. That is if an atom has 5 electrons in its outer shell then it has 3 unpaired electrons.
How Many Electrons Does Magnesium Have Quora
Two valence electrons are present in Group 2s element.
. Potassium has an atomic number of 19 which means one neutral atom of this element has 19 electrons. Does carbon have 4 or 8 valence electrons. How many valence electrons are in an atom of.
Because that the key group elements not shift metals the group number tells united state the number of valence electrons in. 3 How do you find the number of valence electrons and shells. The electron configuration shows that the magnesium atom has acquired the electron configuration of neon.
5 Where are the valence electrons in barium. 13 How many unpaired electrons does MN 4 have. In a neutral atom ie.
Helium only has 2 electrons and therefore it has. Neutron proton and. 11 How many valence electrons are in a fluorine atom and a fluoride ion quizlet.
A car speeds up from 10 ms to 24 ms. 7 What is valence electron of F. Thusmagnesium has actually two valence electrons.
Magnesium has two valence electrons. Magnesium is a form 12 and is classified in Group 2 of the Periodic Table. Place the electrons in shells until you have used up all of the electrons.
5 How do you find the valence shell of an atom. An element in Group 2 has two valence electrons. The valency of magnesium is 2.
Find an answer to your question How many valence electrons does magnesium Mg have. 94 33 ratings Neutral - iron - 55. The atom of sodium has 11 electrons 11 protons along with 12 neutrons but Na contains one less electron 11 protons along with 12 neutrons as the ion has lost 1 electronIt makes the electrons present in that atom equivalent to the nearest gas which is Noble which is Neon and has 10 electrons.
Magnesium has two valence electrons. They each have two electrons in their outer shell. A 1 B 2 C 3 shelbycg02 shelbycg02 12012017 Physics High School answered How many valence electrons does magnesium Mg have.
15 How many valence does El have. 9 How many 4s electrons are in GE. How many protons neutrons and electrons are in a neutral atom of 56Fe.
It an atom has 12 electrons and is neutral then it must be Mg magnesium and magnesium has 2 valence electrons which would be used in bonding. 10 How many energy levels and valence electrons does an atom of germanium Ge have. Tc- 97 has an atomic number Z 43 and Atomic mass A 97.
4 How many electrons will there be in the outer energy level shell of an atom X. 9 How many valence Does CA have. 6 How Valency is barium 2.
9 Which of the following has two unpaired electron. 13 How many valence electrons does xenon have. Does Magnesium Have 10 Valence Electrons.
10 How many valence electrons does the ion F 1 have. It is electrically neutral with 2 electrons in its outer valence shell. 5 Does fluorine have 5 or 7 valence electrons.
2 How many electrons are in the valence shell of each atom. It an atom has 12 electrons and is neutral then it must be Mg magnesium and magnesium has 2 valence electrons which would be used in bonding. 2 Why does barium have 2 valence electrons.
12 What group is. How Many Valence Electrons Does A Magnesium. 11 What is meant by unpaired electrons.
Thus magnesium has two valence electrons. Magnesium has a atomic number of 12 and has a total. 12 How many neutrons does GE have.
8 How many energy levels does Mg have. Valence electrons are the electrons found in an atoms outer energy level. For a neutral atom the number of electrons is the same as the number of protons.
What is the atomic number of this atom. The total number of electrons present in the valence shell of an atom are called valence electrons and there are a total of two electrons present in the valence shell of magnesium 3s 2. 4 How many electrons and valence electrons does barium have.
Mg2 is an ion of magnesium that has given up its 2 outer shell electrons. Magnesium is element 12 and belongs to Group 2 of the Periodic Table. Since the last shell of a magnesium-ion has eight electrons the valence electrons of magnesium ion Mg 2 are eight.
Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom. How Many Electrons Are In Its Valence Shell. 14 How many.
7 How many valence electrons does group 1 have. 11 How many 4p electrons are in GE. The number of electrons for neutral atoms would be equal to the atomic number for the element.
Magnesium has actually 2 valence electrons. 12 How many unpaired electron are present in N2. 14 What is the orbital notation of GE.
Mgs electron configuration is also known as Ne3s-Does Magnesium Have 12 Electrons. Therefore the number of electrons in neutral atom of Magnesium is 12. Magnesium has two valence electrons.
6 How do you find the valence of fluorine. 9 How many valence does El have. An element in group 7 has 7 electrons in its outer shell.
3 What is the valence electron of barium. Also the electron configuration of Mg is 1s² 2s²2p⁶ 3s² or Ne3s². Since the 3s² electrons are the outermost electrons magnesium has two valence electrons.
8 Does Barium have 6 valence electrons. The full number ofelectrons current in the valence covering of one atom are referred to as valence electronsand there room a total of two electrons present in the valence shell of magnesium3s2. Answer and also Explanation.
10 What is the electron configuration of magnesium. 7 How many valence electrons does magnesium have. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus.
This means that the ionized form Mg2 has two more electrons than protons and thus carries an overall charge of minus 2e. 8 Why does C have 4 valence electrons. 6 How many valence electrons does.
1 Atom X Has 17 Protons. 8 What is the valence of fluorine. That is in this case the valence valency of the magnesium-ion is 2.
Magnesium is a form 12 and is classified in Group 2 of the Periodic Table. The free element however is a neutral atom and does not carry any charge since it has no electrons to give up nor a positive nucleus to take them.
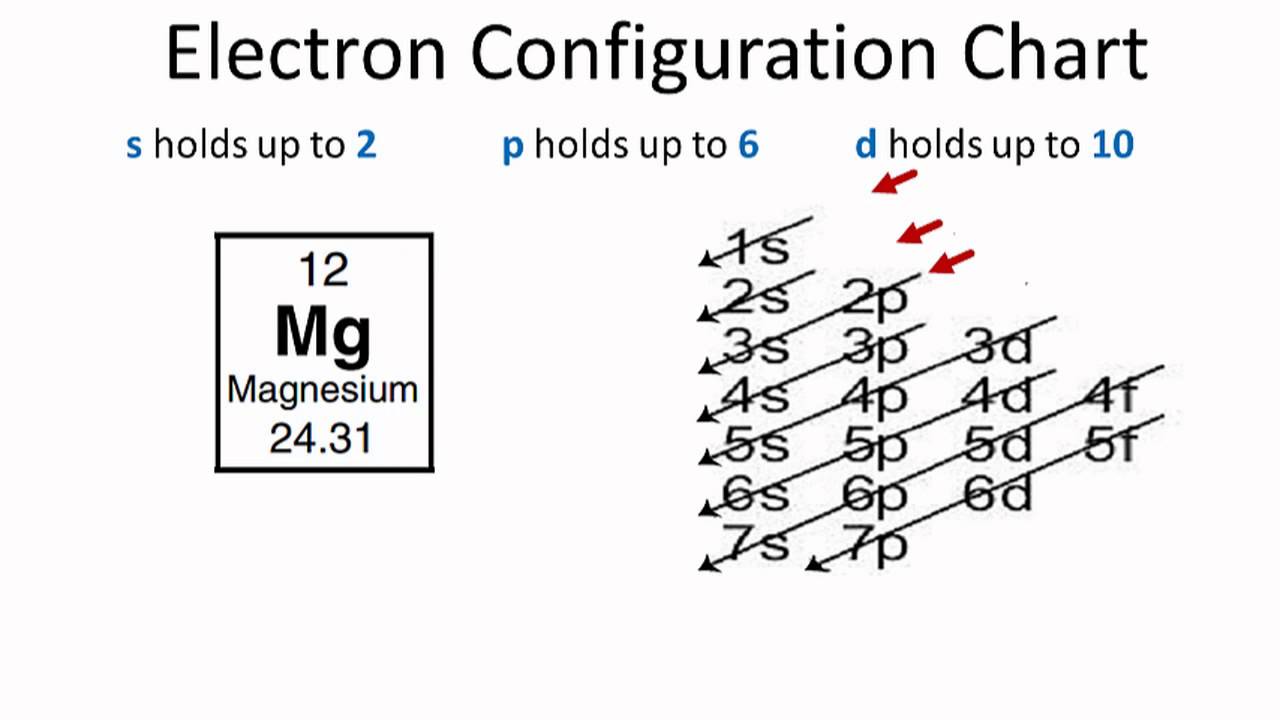
Electron Configuration For Magnesium Mg

How Many Valence Electrons Does Magnesium Mg Have Valency Of Magnesium
No comments for "How Many Valence Electrons Does a Neutral Magnesium Atom Have"
Post a Comment